Project 1 - Activities characteristics
Project 1 covers the HCV screening for the general population and research part requiring the collection of epidemiological data. The Project’s duration (involving the preparation and analysis of collected data) accounts for 4 years (2012-2016). The HCV screening will be conducted in the years 2013-2014.
Collaborating sites and geographical range
The Project is executed in 10 voivodeships in primary health care units localized in urban and rural areas. The voivodeships enrolled on the Project are: dolnośląskie, kujawsko-pomorskie, lubelskie, łódzkie, małopolskie, mazowieckie, podlaskie, podkarpackie, pomorskie and zachodniopomorskie. In each voivodeship, 3-5 primary care units, which were selected randomly from the Registry of Healthcare Centers, participate in the Project.
Internal trainings
The internal trainings concerning the Project’s procedure precede the Project’s execution in randomly selected primary care units. The personnel of enrolled primary care units was also invited to participate in trainings covering expert’s lectures on epidemiology and prevention of HCV infections, diagnostics of HCV infections, treatment of patient infected with HCV and advances observed in hepatitis C treatment which were organized within the frames of Project 3 or Project 1.
Screening and collection of data
It was assumed that in each healthcare unit approximately 400 persons will be subject to testing, ranging from 200 to 800 depending on interest and capabilities of the units as well as the total number of tests offered in each voivodeship. The laboratory investigations are held in Department of Virology of the National Institute of Public Health – National Institute of Hygiene (NIPH-NIH).
The Project will be executed according to the following scheme (Fig. 1).
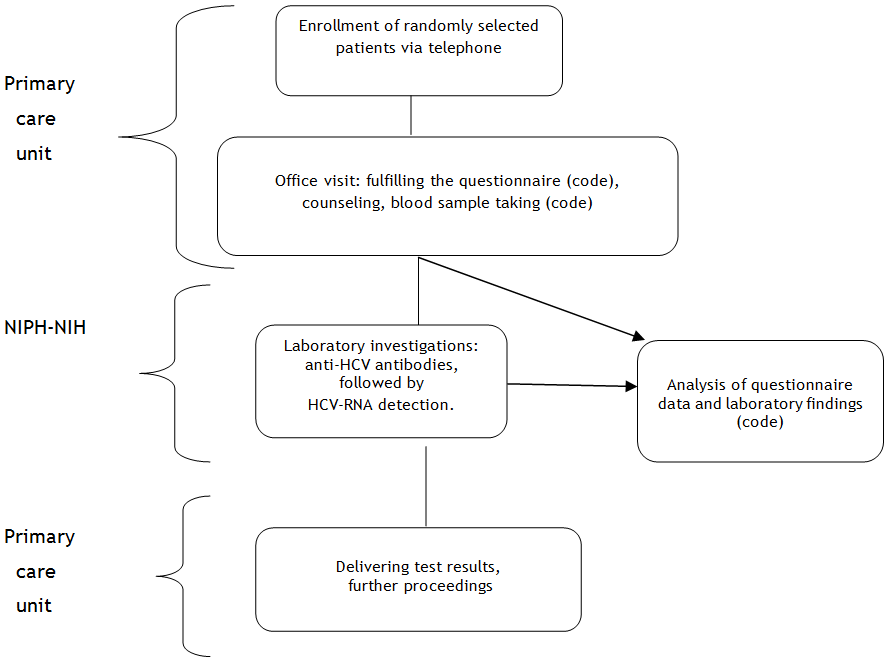
Given the high prevalence of possible exposures in the population and requirement to select representative group for research purposes, the persons will be chosen on a random basis from the register of patients of particular units. The selected persons will be invited via telephone and the date of office visit will be established.
During the visit each patient will donate venous blood sample, fill the questionnaire, and will receive a brochure and will have the chance to ask questions regarding infection with hepatitis C virus.
In order to ensure the comparability of testing, the serum samples will be processed in the Department of Virology of NIPH-NIH. All the patients will be subject to anti-HCV testing. The test to detect the presence of virus (HCV RNA), confirming the infection, will be performed in patient with positive anti-HCV test results.
The serum which remained after testing will be stored in the serum bank. All the information enabling to link the person with his/ her sample will be deleted. Based on a patient’s informed consent, the serum will be stored with a purpose to conduct further studies on epidemiology of communicable diseases in the general population in Poland.
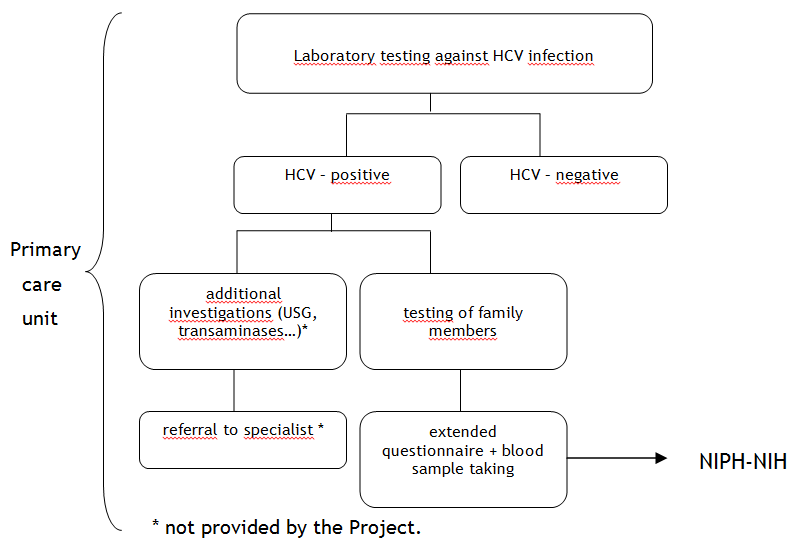
The laboratory test results will be sent to the primary care units. Each patient will receive appropriate information concerning further proceedings (Fig. 2). The HCV-positive persons will be provided with the offer to test his/ her family members and sexual partners against HCV within the frames of the Project.
Data analysis and development of report
The questionnaires, which were anonymously fulfilled by the Project’s participants and marked with the codes will be sent to the Department of Epidemiology of NIPH-NIH. The coded laboratory test results will also be delivered to the Department of Epidemiology.
The database will be used for analysis of hepatitis C epidemiology in Poland, including the prevalence of HCV infections in the general population, frequency of well-known risk factors of HCV infections and risk factors observed in household settings. The analysis will be supplemented with the conclusions and observations of personnel conducting the study in primary health care units which will be collected during monitoring visits.
The final stage of the Project will consist in developing the report involving the recommendations of HCV testing in the general population (indications, frequency etc.).